EJECTOR HYDROGENIZATION Division
Hydrogenization is one of the most applied procedures in organic synthesis and in industrial applications it is the most used reaction. The hydrogen molecule can enter the reaction faster with more functional groups from any other molecule. Hydrogenization compared to alternative methods for reduction is the most economic. When viewing general trends in hydrogenization technology it can be established that greater attention is being paid to:
- optimization of development laboratories and semi-industrial research,
- increase in economy,
- improvement of safety of operation and the environment.
The stated development trends are most efficiently fulfilled using reactors with circulatory flow and an ejector mixer. These types of reactors are applied more widely in many chemical processes, but are most often used for hydrogenization.
The operating principle of a filling hydrogenization procedure using ejectors is given in figure 11.1. The starting material (can be dissolved in a solute or not or in the form of a fine solid catalyzer) is pumped in from the reactor and then via a heat exchanger (if heating or cooling is needed) and ejector is put back into the reactor forming a circular circulation.
When passing through the ejector nozzle the rate of the reaction suspension increases so it enters the chamber at a great rate. In the ejector chamber particles of the reaction suspension collide with the gas particles present (hydrogen) and both fluids are reduced to the smallest particles forming a completely fine homogenous mixture.
The reduced fine particles of both fluids have a large active touching surface that enables fast energy exchange (mechanical, heat and chemical) and depending on the fluid type a relatively fast reaction, i.e. absorption.
The formed gas and suspension mixture is thrusted out of the ejector into the reactor space filled with the suspension. The undissolved, i.e. unreacted excess hydrogen (gas) after leaving the ejector lifts to the suspension surface in the gas area of the reactor fr4om where it is pumped in again by the reactor.
Experiments have established that the ratio between the surface mass transfer and reaction volume per energy unit (m2/m3) is two times higher than the one reached in a reactor with a turbine mixer, thus significantly increasing the reaction rate.
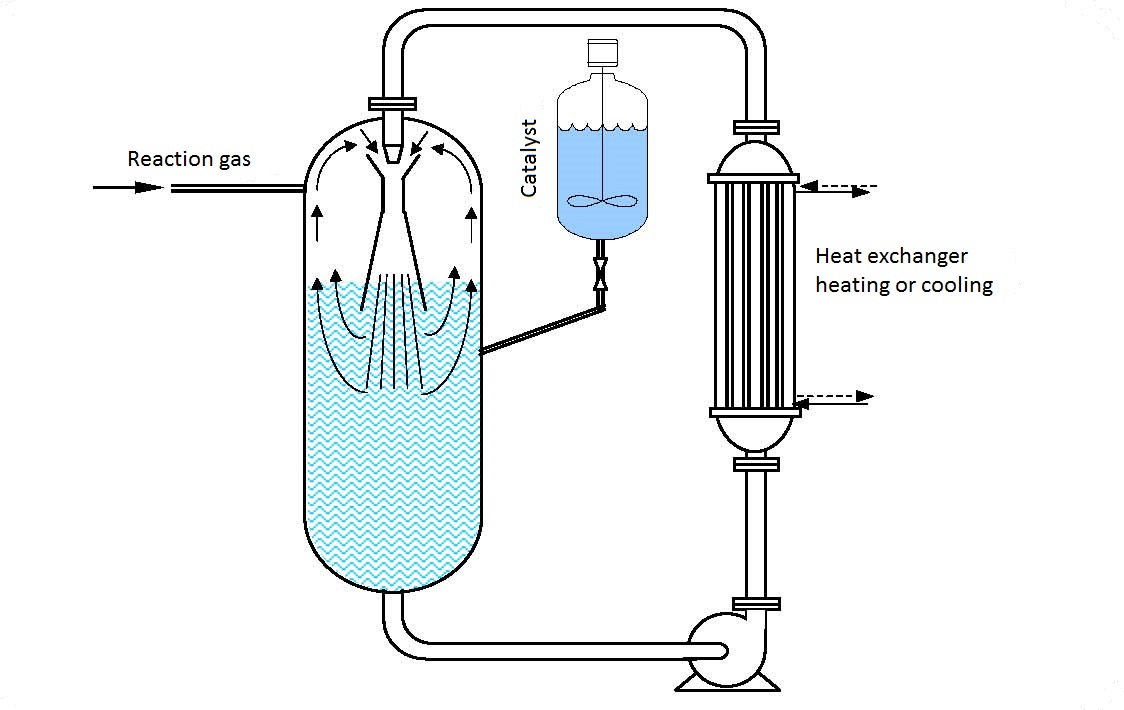 |
| Figure 11.1 Hydrogenization |
In a reactor with a circular flow the degree of gas-liquid mixing is constant during the reaction that has a significant influence on shortening the time required for the reaction and also reduction of the energy utilized per production unit.
A great advantage of a circular reactor is that the heat exchanger is installed outside the autoclave so there are no dimensional limitations. Selection of a corresponding exchanger size with a large heat transfer coefficient enables fast removal or conveyal of heat necessary for the reaction.
A system of automated temperature control enables maintenance of the reaction temperature with a precision higher than ±1oC. Such a system enables maintenance of a homogenous mixture during the whole mixing time.
A combination of a fast reaction rate and precise temperature control in most cases leads to the best possible yield and prevention of side reactions. Due to the more intensive and shorter mixing time and uniform catalizer distribution, the percentage of the catalizer introduced into the reaction is reduced for 30-50% and more in some cases, so its total consumption is reduced.
Circular reactors with an ejector have a series of advantages compared to reactors with mixers and they are:
- high removal and conveyal heat rate,
- lower catalyzer use, per production unit,
- faster reaction,
- savings in driving energy of mass transfer and thus a shorter working cycle,
- no mixer requiring large driving engines and a strong construction,
- ejectors have no mobile parts so do not require lubrication and maintenance,
- ejectors can relatively easily be installed and put into operation indenepndently of a mixer or work in parallel with the mixer,
- the process can easily be automated, with a precise temperature control enabling a physically easier, safer and secureroperation.
Application:
Hydrogen catalytic hydrogenization is applied in the production of cooking oil, chemical industry or organic intermediaries, dyes, solutions, agrochemistry, pharmacy and many other technological processes.
The standard reactions applied are:
- heterogeneous catalytic hydrogenization,
- acryling,
- nitrilation,
- phosgenization,
- amilation,
- chlorination,
- carbonization,
- etoxilation and
- many other reactions.
Note:
An ejector can additionally be installed in many existing reactors with mixers and it can work in parallel with the mixer or independently. Ejector installation is simple and does not require any changes in the existing reactor.