EJECTOR VENTILATORS Division
Depending on the driving fluid ejector ventilators can be:
- ejector gas ventilators (gas-gas);
- ejector vapor ventilators (vapor-gas);
- ejector hydro ventilators (liquid-gas).
All ejector ventilators use energy of the input fluid for drive:
- gas (most often air);
- liquid (most often water) and
- vapor (most often water vapor).
The operating principle of all ejectors is to transform most of the thrusting energy of the driving fluid, by passing through the ejector nozzle, into kinetic (speed) energy, forming a jet flow that enters the ejector chamber at a great speed.
In the ejector chamber particles of the driving fluid come into contact with particles of the pumped gas, they collide and mix with them, transferring part of their kinetic energy, thus slowing down, while particles of the pumped fluid receive part of the energy and thus accelerate, forming a fine homogenous mixture that flows towards the diffuser. Due to the great difference in flow rates of the driving fluid that for gas and vapor is above the speed of sound, collision and destruction of the pumped fluid into the smallest particles occurs, forming large interacting surfaces. These large interacting surfaces enable very fast energy (mechanical, heat and chemical) exchange.
Neighbouring particles from the input pipeline occupy spots vacated by the taken up particles of the pumped fluid forming a flow of pumped gas towards the ejector.
After leaving the chamber the homogenous mixture passes through the diffuser in which the current flow expands thus its rate decreases and the pressure rises so the pressure required for overcoming resistance in the thrusting pipeline is achieved at the ejector output.
5.1 Ejector gas ventilators
These ventilators use gas (air) from the compressor, jets or air from ventilators (centrifugal and axial) as the driving fluid.
Application:
They are used for small and large flows (several thousand m3/h), for ratios of output to pumped pressure p3/p2 ≤ 1.2, for low and high temperatures. They are used for extracting polluted air, gas and vapor from working and storage spaces, for de-aeration of reactors, mixers and other aggregates in the chemical industry, for ventilation of vessels, ships and flues, for gas burners, also in the leather, textile and tobacco industry.
Their special advantage compared to classical ventilators is that the mass or volume ratio between the pumped (polluted) and driving (clean neutral) gas can be realized in desired ranges, enabling formation of a non-explosive, non-inflammable, non-toxic and non-aggressive mixture that can be safely transported and deposited without any harmful consequences.
Such devices secure the safest transport of explosive and inflammable gases as they have no mobile parts and do not use electric energy so no sparks are possible.
Ejector ventilators can have several serially connected nozzles (figure 5.1). Such a nozzle set-up enables increase of the driving gas flow for the following nozzle, thus increasing the flow of pumped gas, i.e. enables maximal utilization of the high pressure of the driving gas.
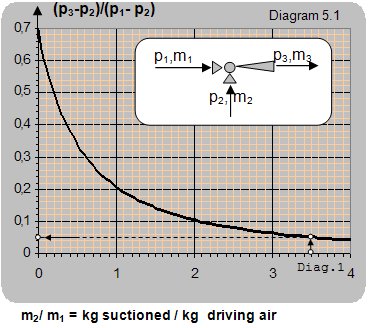
If the ratio between the input and pumped pressure p3/p3 ≤ 1.2 and the ratio between the driving and pumped pressure p1/p2 ≤ 1.25 centrifugal ventilators can be used to provide air driving. The curve showing the relation between the pressure ratio and the mass flow ratio is given in diagram 5.1. If the pressure ratio p3/p3 ≤ 1.2 and p1/p2 ≥ 1.25 then compressed air from a compressor and jets can be used for providing driving air (curves showing the relation between the pressure ratio and mass flow ratio are given in diagram 5.2).
Example 5.1
Data: The driving air pressure p1 = 50 mbar (1.050 barabs), pressure at the ejector output p3 = 20 mbar (1.020 barabs) and mass flow ratio of m2/m1 = 3.5.
How big a pumping sub-pressure can be attained at the ejector input?
Solution: For the mass flow ratio of m2/m1 = 3.5, the pressure ratio is read from diagram 5.1 as: (p3-p2)/(p1-p2) = 0.005 from which follows p2 = (p3 – 0.05×p1)/0.95 = 18.4 mbar.
 |
Example 5.2
Data:Pressure of the driving compressed air is p1 = 3 bar (4 barabs), pressure at the ejector output is p3 = 30 mbar (1.030 barabs) and pumped pressure p2 = 1 bar (atmospheric pressure).
How many kilograms of polluted air can be pumped in with 1 kg of compressed air?
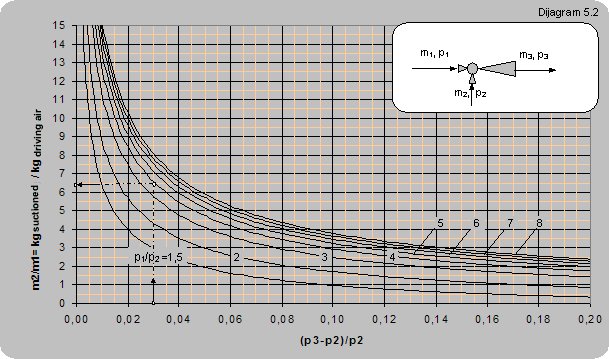 |
Solution:
For (p3-p2)/p2 = (1.030-1)/1 and p1/p2 = 4/1 = 4 one can read m2/m1 = 6.5.
So, one kilogram of compressed air can pump in 6.4 kg of polluted air.
5.2. Ejector vapor ventilators
These ventilators use saturated or overheated water vapor with a low, medium and high pressure as the driving fluid. They are used for pumping in and thrusting air, gas and vapor for pressure ratios p3/p2 ≤ 1.2 and pumped gas flow of several thousand m3/h.
Application:
They are used for extracting air, polluted gas and vapor from working and storage areas, for ventilation of vessels, ship cisterns, air moisturizing circulation in the leather, textile and tobacco industry and other fields of technological processes.
If overheated water vapor is used as the driving fluid the pumped air and gas can be dried, while if saturated water vapor is used the pumped air and gas can be moisturized.
When ejector vapor ventilators are used only for air moisturizing, where increase of the pumped air pressure is not necessary a very favorable flow ratio can be achieved (higher than 100 m3 of air per 1 kg of water vapor). Such a procedure avoids mist formation, even for relatively high moisture of the pumped air.
5.3. Ejector hydro-ventilators
These ventilators most often use water as the driving fluid. Liquid under pressure when passing through the jet transforms the thrusting energy into kinetic energy so at the jet output it enters the mixing chamber at a great rate. In the mixing chamber the liquid flow takes up air, gas and vapor and via a chamber and diffuser expels them into the atmosphere or a thrusting pipeline. Due to the large difference in flow rates of the driving liquid and pumped gas and vapor both fluid mix and destruct into the smallest particles. Such well mixed fluids with large mutual contacts enable fast separation of all types of solid particles from the pumped fluid. Gas and vapor are partially dissolved in the liquid and a reaction occurs if the conditions are right.
At the ejector output the liquid is via deposits let out into the sewer or recirculated and the washed and purified gases are further utilized or released into the atmosphere. The driving liquid is most often recirculated and renewed when required.
Application:
They are used for pumping and extracting polluted gas and vapor from working and storage spaces, ship cisterns, reactors, mixers and other vessels and devices in the chemical industry, also for air circulation in the tobacco, leather and textile industry. Their special application is extraction of inflammable and explosive gas and acid and poisonous vapor. Gases exiting the ejector have relative moisture of 90 to 1000.
They are used for pumping gas at different temperatures for volume ratios between the pumped gas and driving liquid of 4 (Vg/Qt, m3/m3pumped gas/liquid). For extraction of gas and vapor from vessels with high vacuum (see Ejector gas vacuum pumps) and compression of gas under high pressure (see Ejector hydro-gas compressors).
Diagram 5.3 shows the dependence between the pressure ratio and volume flow of air and water.
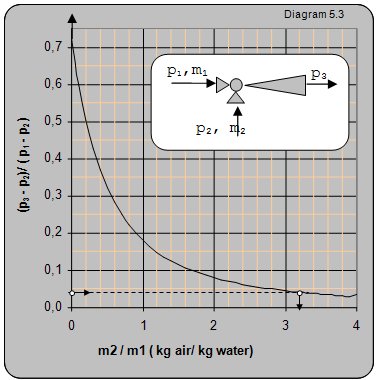 |
Example 5.3
Data:The pressure of the driving water entering the ejector p1 = 4 bar (5 barabs), pressure of the air and water mixture at the ejector output p3 = 0 bar (1.2 barabs), pressure of the pumped air is atmospheric (1 barabs).How many kilograms of water will be pumped in with one kilogram of air?
Solution: (p3-p2)/(p2-p1) = (1.2-1)/(5-1) = 0.04. From diagram 5.3 m2/m1 = 3.2 that means that 3.2 m3 can be pumped in with 1 m3 of water.
When ordering all three pressures p1, p2 and p3 need to be stated or the mass ratio between air and water flow m2/m1 and two of the three stated pressures.
 |
| Figure 5.1 Different types of construction ejector fan |